BALVERSA® was studied in 266 people with advanced bladder cancer who had tried immunotherapy medicine

BALVERSA® may help you live longer and shrink tumors
Patients lived longer on BALVERSA® compared with chemotherapy
- Median overall survival with BALVERSA® was 12.1 months vs 7.8 months with chemotherapy
More people taking BALVERSA® saw their tumor shrink compared with chemotherapy
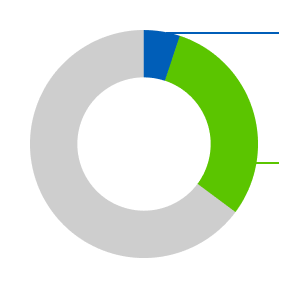
5.1% of people had their tumor disappear completely
(known as a complete response), compared with 0.8%
of people on chemotherapy
30.1% of people had their tumor partially shrink (known as a partial response), compared with 7.7% of people on chemotherapy
5.1% of people had their tumor disappear completely (known as a complete response), compared with 0.8% of people on chemotherapy
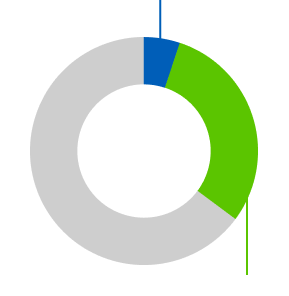
30.1% of people had their tumor partially shrink (known as a partial response), compared with 7.7% of people on chemotherapy
Remember, each person is different, so the results you see with BALVERSA® may not be the same as in the study.

